Sea Water Injection System
| ✅ Paper Type: Free Essay | ✅ Subject: Engineering |
| ✅ Wordcount: 1730 words | ✅ Published: 31 Aug 2017 |
In order to improve the oil recovery in an oil field the injection of sea water is used to increase the pressure inside the reservoir and enhance the oil production. The graph given in Figure 1 is a typical seawater injection system, before the injection process, water must have a treatment to decrease the corrosion rate caused by seawater in pipe lines, surface and downhole injection equipments. The treatment is based in a mechanical de-aeration process and chemical scavenger injection to decrease the concentration of oxygen in seawater.
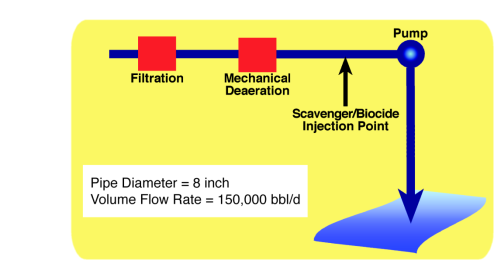
Figure 1. Seawater Injection System
In a normal operation of the seawater injection system the conditions of the process are 150,000 barrels per day, pipe diameter of 8 inches at 25 °C, however in terms of corrosion parameters the data is provided in table 1, this table shows that the mechanical deaeration process reduce the most quantity of Oxygen concentration in seawater.
Table 1. Concentration of Oxygen in normal operation
|
Concentration of O2 in different Units |
|||
|
PPB |
mg/l |
mole/m3 |
|
|
Feed Seawater |
7,000 |
7 |
0.22 |
|
After mechanical de-aeration process |
100 |
0.1 |
0.003 |
|
After scavenger dosage |
10 |
0.01 |
0.0003 |
With the data provided, the corrosion rate in normal operation condition is 0.0454mm/year (the calculation step by step including unit conversion are shown in the appendix) hence the corrosion rate is far less than the company acceptable value which is 0.1 mm/year, and it means that the system is working properly.
It has been found that the mechanical de-aeration equipment requires repair, and it will be out of operation for between one and three months.
Water system Injection without a mechanical de-aeration process:
Calculating the limit current density with the following equation:
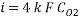
Concentration of Oxygen only with the addition of Scavenger dosing 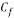 = 6.910 ppm
= 6.910 ppm
K is the mass transfer coefficient and it calculations and unit conversions are shown in the appendix.
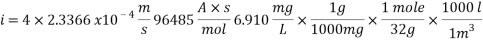
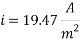
Calculating the Corrosion Rate:
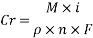
Assuming the main component of the pipe Iron therefore n= 2
M= 55.84 g/mol
Density: 7.87 g/cm3
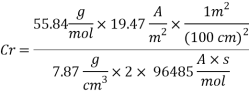
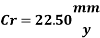
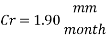
Now we can compare the corrosion rate of each case and determine the implications of operating the system without the mechanical de-aeration.
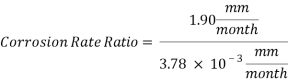
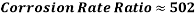
Hence the corrosion rate will increase 502 times without the mechanical de-aeration. Based on this result it is obvious that the most important process for oxygen removal is the mechanical deareation.
The company request the assessment in a technically and economically point of view three operational solutions during the repair of the mechanical de-aeration equipment.
For the given acceptable corrosion rate less than 0.1 mm/year, a corrosion rate value of 0.09 mm/year was used to calculate the implications of the possible solutions.
a. Decreasing the Flow Rate
Assuming an acceptable corrosion Rate of 0.09 mm/year,
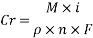
From the equation above we can reach the Current density:
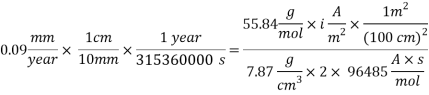
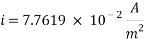
With current density we can reach mass transfer coefficient k:
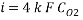
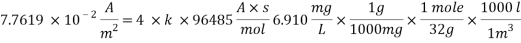
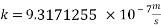
Now we can reach the new Sh number:
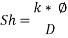
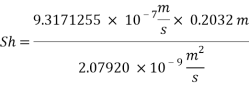
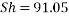
With Sh number we can obtain Re number:
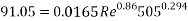
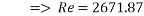
This value of Reynolds number means that we are in the transition regime between laminar flow and turbulent flow.
Now we can reach the flow rate:
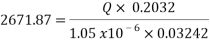
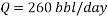
From the technical point of view and based on the concepts of fluids mechanics, decreasing the flow volume to 260 barrels per day will generate a laminar flow (Re less than 3000) on the pipe, in other words it means that the velocity will not be in the required optimum range of 1.5 m/s to 2.5 m/s, according to Streeter. Doing the calculations the velocity will reach a very slow value of 0.014 m/s in the pipe, which is by far lower than the minimum value of 1 m/s. Therefore technically, the reduction of flow rate to reach an acceptable corrosion rate is not a possible solution.
In addition, this kind of diminution of the flow rate (577 times lower than the original) would have impacts on the oil well. Specifically, it would not be able to maintain the pressure at the desired level and therefore would have a big impact on oil production leading to money loss.
b. Increasing the amount of scavenger
Assuming an acceptable corrosion Rate of 0.09 mm/year
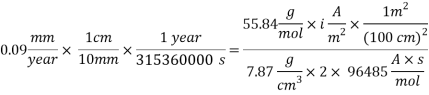
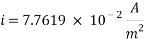
From the equation below we can reach the concentration of Oxygen that we need to contain in the water in order to have an acceptable corrosion rate
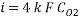
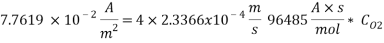
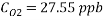
Using Sodium sulfate as scavenger the following reaction will proceed:
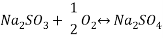
Hence form the stoichiometry of the reaction the relation between the compounds will be 2 moles of Scavenger and 1 mole of Oxygen.
Therefore the amount of scavenger Sodium sulfate needed is: 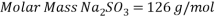 =>
=> 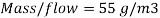
In an injection flow rate of 150,000 bbl/day
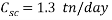
Based on scavenger’s calculations we need to provide the system with a high amount of scavenger to reduce the oxygen concentration that gives an acceptable corrosion rate, it is up to 1.3 ton per day, it is nearly 80 times more than amount of scavenger used in normal operation, which is about 17 kg per day.
On the economically point of view, if the scavenger will substitute mechanical de-aeration for a month, the need of scavenger will be approximately 40 ton per month. By using the commercial price of scavenger 0.64 USD/kg (https://www.icis.com), it will cost around 832 USD/day and scaling it to a month it will cost nearly 24,960 USD/month.
c. Corrosion Inhibitor
Corrosion inhibitor compound will reduce the corrosion rate by preventing both anodic and cathodic reactions. Anodic inhibitor will be adsorbed onto metal surface to form protective film and prevent metal dissolution while cathodic inhibitor will minimize O2 reduction reaction by forming non-conducting film on metal surface. And in technical terms it could be the solution of the problem. However, from the calculations, we know that corrosion rate without the mechanical deareation is 22.5 mm/year and the aim is to decrease the corrosion rate below 0.1 mm/year. Based on the corrosion inhibitor risk category that is proposed by Hedges (2000), if the expected uninhibited corrosion rate is graeter than 6 mm/year inhibition is unlikely to provide integrity for the full field life. Therefore corrosion control of the system could not be efficient with a only corrosion inhibitor because of the high requirement of availability.
Based on the results of the three possible options, on the economically point of view decreasing the injection flow rate will impact in the production of oil, and decreasing the main product (oil) of the industry it will have terrible effects in the oil company. Therefore in the corrosion engineering point of view the most accurately solution is to increase the amount of scavenger (Na2SO3) in order to reach a corrosion rate of 6 mm/year and then with the addition of corrosion inhibitors the corrosion rate can be reduce to an acceptable value of less than 0.1 mm/year.
The dosage of O2 scavenger has to be interrupted for 8 hours per week for the injection of the biocide. During this time if there was not the corrosion inhibitor, the Corrosion rate would be 22.5 mm/yr. but if the Corrosion inhibitor inhibition rate is 98.5% (as from 6mm/yr to 0.09mm/yr), the corrosion rate would be:
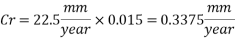
Therefore the Corrosion rate would be: 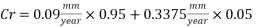
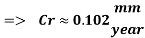
The Corrosion rate is slightly above the required norm (0.002 mm/yr), but in the worst case scenario, 3 months with no deaerator, due to the fact the Corrosion rate with the mechanical deaerator is 0.0454 mm/yr which is 0.0546 mm/year less than the required standard. So, in a year perspective the slightly more amount of Corrosion will be not significant and the system will work properly.
Also, the amount of Na2SO3 needed to reach a CR of 6 mm/year is 905 Kg/day and it will cost around 580 USD/day.
Finally, in order to choose the ideal corrosion inhibitor laboratory tests must be performed in the same seawater that will be used. In situ tests would help to assure the quality of the results.
- Streeter, Victor L. “Handbook of fluid mechanics.” McGraw-Hill, ed 1 (1961).
- Hedges, B. (2000) “The Corrosion Inhibitor Availability Model“, NACE International, Paper 00034.
- Water system Injection with a mechanical de-aeration process and Oxygen scavenger addition:
Considering the water system injection above and the following data we can reach a corrosion rate value in the next steps.
Data provided:
- Pipe Diameter:
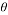 = 8 inch, therefore the Area A = 50.26 in2 = 0.032429 m2
= 8 inch, therefore the Area A = 50.26 in2 = 0.032429 m2 - Volume Flow Rate:
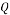 = 150000 bbl/d
= 150000 bbl/d - Initial Oxygen Concentration
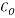 = 7 ppm
= 7 ppm - Concentration of Oxygen After Mechanical De-aeretion
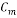 = 100 ppb
= 100 ppb - Concentration of Oxygen After Scavenger dosing
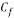 = 10 ppb
= 10 ppb - Kinematic Viscosity:
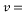 1.05 X 10-6 m2/s
1.05 X 10-6 m2/s - Schmidt number
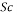 = 505
= 505
- Calculation of Re number:
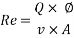
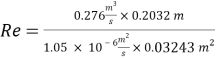
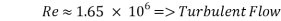
- Calculation of Sh Number:
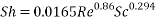
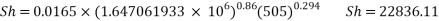
- In turbulent Flow calculation of Diffusion coefficient:
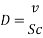
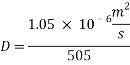
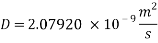
From Sh number we can reach the mass transfer coefficient k:
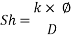
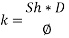
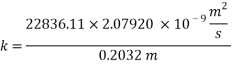
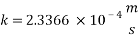
Now calculating the limit current density with the following equation:
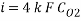
Concentration of Oxygen after mechanical de-aeration and Scavenger dosing 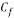 = 0.01 ppm
= 0.01 ppm
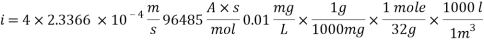
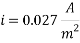
Calculating the Corrosion Rate:
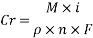
Assuming the main component of the pipe Iron therefore:
n= 2
MFe: 55.84 g/mol
Ï: 7.87 g/cm3
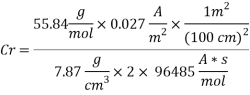
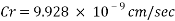
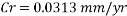
For 8 hours per week, the O2 scavenger dosing is interrupted for biocide to be injected. So, there is an Availability of : 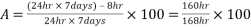
=> 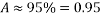
The Corrosion rate at a concentration of 0.1ppm of O2 is:

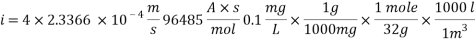
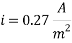
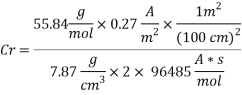
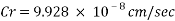
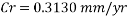
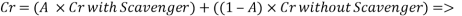
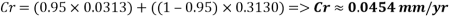
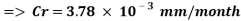
- Water system Injection without a mechanical de-aeration process:
Calculating the limit current density with the following equation:
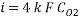
Concentration of Oxygen only with the addition of Scavenger dosing 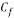 = 6.910 ppm
= 6.910 ppm
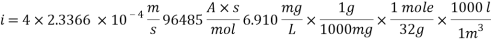
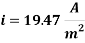
Calculating the Corrosion Rate:
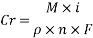
Assuming the main component of the pipe Iron therefore n= 2
M= 55.84 g/mol
Density: 7.87 g/cm3
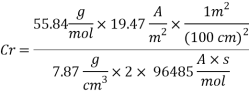
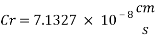
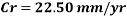
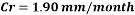
Now we can compare the corrosion rate of each case and determine the implications of operating the system without the mechanical de-aeration.
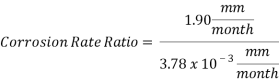
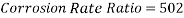
Hence the corrosion rate will increase 502 times without the mechanical de-aeration.
- Evaluation of the following operational solutions:
- Decrease the flow rate of water:
Assuming an acceptable corrosion Rate of 0.09 mm/year
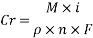
From the equation above we can reach the Current density:
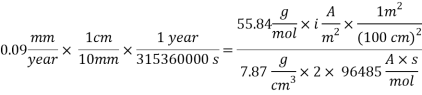
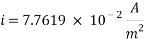
With current density we can reach mass transfer coefficient k
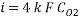

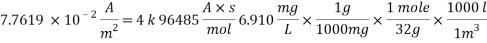
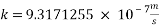
Now we can reach the new Sh number:
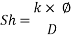
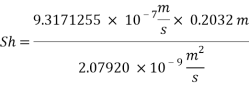
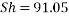
With Sh number we can obtain Re number:
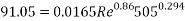
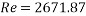
This value of Reynolds number means that we are in the transition regime between laminar flow and turbulent flow.
Now we can reach the flow rate:
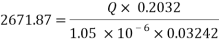
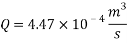
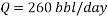
- Increasing the amount of scavenger:
Assuming an acceptable corrosion Rate of 0.09 mm/year
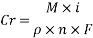
From the equation above we can reach the Current density:
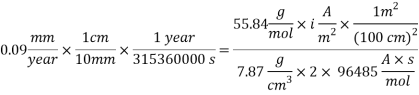
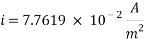
From the equation below we can reach the concentration of Oxygen that we need to contain in the water in order to have an acceptable corrosion rate
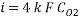
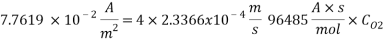
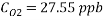
Using Sodium sulfate as scavenger the following reaction will proceed:
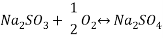
Hence form the stoichiometry of the reaction the relation between the compounds will be 2 moles of Scavenger and 1 mole of Oxygen.
Therefore the amount of scavenger Sodium sulfate needed is:
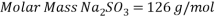
Hence we need: 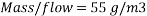
In an injection flow rate of 150000 barrels per day
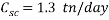
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


