ISO 45001 occupational health and safety standard
| ✅ Paper Type: Free Essay | ✅ Subject: Nutrition |
| ✅ Wordcount: 1827 words | ✅ Published: 23 Sep 2019 |
ISO 45001 occupational health and safety standard
Introduction:
occupational health and safety are also referred to workplace health and safety concerned with safety health and welfare of people at work, it is used to reduce workplace risks injuries and accidents to create better and safe workplace. The goal of this standard is to include safe and healthy work environment. It provides an effective process for improving work safety. It is designed to help organization to reduce workplace illness and injuries
Interpretation:
Certification to ISO 45001 is not a requirement of the standard but is useful tool to show that you meet its criteria. Certification is given to companies who have great work place. OH&S certification requires organization to manage and monitor hazards that may harm employees but also control risks and create improvements it also benefits the performance of company.
Benefits of certification are
- Reducing hidden costs of workplace accidents and incidents
- It leads to continues improvement
- It reduces and manages risks
- It increases communication
- It gives a greater control over processes and internal system
Discussion:
According to international labour organization (ILO) Over 7 600 people die each day from work-related accidents or diseases that’s over 2.78 million every year. Health and safety are number one concern of most companies and business, still deaths and injuries occur. (OH&S) aims to help organization to reduce workplace risks and create better and safe workplace. This is to achieve to control all factors that result injury, illness and death cases
ISO 45001 is worlds first international standard that deals with health and safety at work
ISO 45001 provides governmental agencies, industry usable guidance for improving worker safety worldwide.
The new OH&S is based on all of ISO management systems which provide guidelines for organizations to plan in how to minimize risk of harm in work place.
OH&S has grown increasingly complicated with many of today’s businesses crossing national boundaries.
The top management must part a role to promote a positive culture and communicate. What needs to be done and why is it important. Senior leaders are involved and integrate OH&S management system into the overall business processes. “ISO 45001 means more focus on leadership and worker participation.
ISO 22000 Food and safety standard
Introduction:
ISO 22000 is related to food and food safety, its aim is to lower foodborne illness, improvement of food safety to reduce contamination and it defines organization to control food safety hazards and ensure food Is safe for consumption. Food safety is a discipline in handling, preparation of food and storage of food. This prevents food illness that result from ingestion of common food that can lead to health hazards. Food safety includes practices of food labeling, food hygiene and give guidelines for management of food in export and import
Interpretation:
ISO 22000 certification is a third- party verification that product meet food safety standards, it is proof of conformity. ISO 22000 certification depends on results of testing inspections that gives confidence to consumer because organizations products are evaluated and accepted by national and international standards.
ISO 22000 conveys to company or business it has successfully met requirements of food safety. The business which are certified will gain competitive advantage in marketplace.
Benefits of certification
- It gives consistency help business to produce safe food and will have quality to met requirements
- It helps management is meeting legal responsibilities in relation to food legislation and regulation and is doing effectively
Discussion:
WHO (wold health organization) reported that about 30% food poising outbreaks in European region in private homes. Annually 76 million cases of food illness that leads to 32500 hospitalization and 5000 deaths. ISO 22000 contributes to ensure food safety hazards throughout the whole food chain from farm-to-table. This becomes essential as hazards occur at any stage of the food chain. Food safety is done by safe food handling by proper handling, proper storing, using sanitary tools, heating cooling properly, transporting by avoid contamination from environment. HACCP plan is used to control food safety from ingredient to production. It not only improves food safety management but also other quality management systems the main benefits of HACCP are:
- It avoids poisoning your customers
- It increases food safety standard
- Organises to produce food safely
ISO 22000 is certifiable standard is one of ISO’s best-known standard.
The goal of food safety professional is to create food safety culture.
ISO 9001 Quality Management
Introduction:
ISO 9001 tells us about how an organization meets the requirements of its customers and other stakeholders affected by its work. ISO 9001 provide guidance and tools for companies and organization who want to ensure that their product or services meet their customer’s requirement and quality is improved. ISO 9001:2015 applies to any organization this organization help industry or company to improve the efficiency of process, to organize processes and continually improve. All organization that use ISO 9001 are transition to ISO 9001:2015
Interpretation:
ISO 9001:2015 certification means that an organization has indicated following
- Follows the guideline of ISO 9001 standard
- Fulfills its own requirements
- Meet customer, regulatory requirements
- Maintains documentation
Certification of ISO 9001 shows that it meets the customers and services requirements.
Organization that use ISO 9001 helps them
- Organize QMS (quality management system)
- Meet necessary statutory and regulatory requirements
- Create employees, management and satisfied customers
- Work efficiently to save costs
- Identify and address the risks
Discussion:
ISO 9001 is based on PDCA (plan do check act) and includes specific topics of
- Responsibilities of management
- Requirement for quality management that includes planning and determine interactions and documented information
- Management of resources that includes organization work environment and human resources
- Product understanding that includes steps from design to delivery
- Analysis, measurement and improvement of QMS through audits
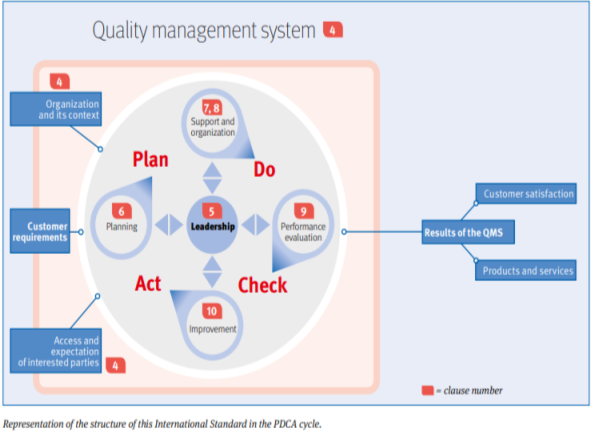
ISO 90001 is based on seven quality management principles (QMPs)
- Customer focus: is to meet customer requirement and exceed customer expectation
- Leadership: it creates unity and engages people in order to achieve organization’s quality
- Engagement of people: it empowers and engage people to be able to create and deliver value
- Process approach: manage and control processes
- Improvement: improving organization performances
- Evidence based decision making: making decision based on analysis, data and information to produce results
- Relationship management: maintaining relationships with interested parties
Implementing and maintaining a quality management system based on ISO 9001
The implementing process is necessary to to achieve benefits of quality management system
There are seven steps that will help to have successful quality management system that fit your organization
- Engage top management to
Agree on why to implement a QMS
Determine customer and parties’ expectations
Understand the quality management principles described in ISO 9000
Define the objectives of the organization
Describe the scope of the QMS
Define the policy
Determine quality objectives
- Identify key processes
Understand ISO 9001 requirements
Determine the risks and opportunities to the processes
- Plan your QMS
Identify controlled processes
Define working environment
Define the skills and facilities
- Document your QMS
Document activities, processes and controls
Prepare information required by standard
Ensure the QMS conforms to ISO 9001 requirements
- Implement your QMS
Manage the processes
measure and monitor equipment
Train employees
Verify processes that are effective
- Manage your QMS
Measure and monitor performance
Focus that customer is satisfied
Perform management review
- Improve your QMS
Seek certification from third-party
Strive for improvement with reference to ISO 9004
Consider implementing business
ISO 9001 must work properly to satisfy customers and achieve improvement through QMS (quality management system). Continual improvement increases effectiveness of organization in order established policy and quality objectives, ISO 9001 requires that you manage and plan processes to improve QMS (Quality management system)
ISO 9001 is used successfully all over the world. In 2013 alone, over one million certificates to the standard were issued across 187 countries,
ISO/IEC 17025 Testing and Calibrating laboratories
Introduction:
ISO/IEC 17025 is a requirement for testing and calibration in laboratories it enables laboratories to have capacity to deliver results and create confidence in their work both nationally and internationally.
ISO/IEC 17025 is helps organization that perform samples, tests and calibration and want valid and reliable results. This includes laboratories, industries, universities, research centers, inspection bodies, product certification organization or other organization that need to do testing sampling or calibration.
The newly version of ISO/IEC 17025 Is ISO/IEC 17025/2017 it allows laboratories to implement quality system and demonstrate competence and produce valid, reliable results. Latest changes of standard encompass activities and new ways of working in laboratories it covers vocabulary, developments in IT techniques and latest version of ISO 9001 on quality management
Interpretation:
To achieve ISO 17025 accreditation, the laboratory QMS (quality management system) and technical competence are assed by third-party. Audits performed regularly to maintain accreditation. accreditation means that laboratory has met technical and management requirements of ISO 17025 and is competent to produce calibrate and test results .ISO 17025 proves that laboratory has met requirements and has ability to provide testing and calibration results.
Benefits for ISO 17025 accreditation
- Control laboratory method variation
- Increase confidence in calibration and testing data
- Greater quality awareness
- Increase in business due to increase in customer confidence and satisfaction
- Save costs due to reduction of re-testing or elimination
- Achieving international recognition and gain in interested parties
- Better control of laboratory operations
- Technical competence of staff
- Maintenance of test equipment
- Quality assurance of test and calibration data
Discussion:
ISO/IEC 17025 version 2005 has five elements which are, scope, Normative references, Terms and definitions, Technical and Management requirements. ISO/IEC 17025 version 2017 is modified and have eight elements that are Scope, Terms and definitions, Normative References, General Requirements, Resources Requirements, Structural Requirements, Process Requirement and Management Requirement.
Structural and General Requirements are interconnected with laboratory organization.
Structure Requirements are issues related plant, people or organization used to produce valid results.
Process requirements are vision of standard that results are based on science and are aimed to be valid technically.
Management system requirements are steps taken by organization to maintain Quality and get valid results.
The two main sections in ISO/IEC 17025:2005 are Management Requirements and Technical Requirements.
Management Requirements are quality control system and documentation control.
Technical Requirements: address the competence of methods, tests, calibrate results, the competence of staff, the type of equipment used in the lab.
Main changes in 2017 version are
- Process approach it matches the newer standards of ISO 9001, ISO/IEC 170211 and ISO 15189.
- Scope is revised and covers calibration, testing and sampling.
- New chapter that introduces Risk based thinking
- Strong focus on information technologies
- Terminology has been updated
ISO/IEC 17025 version 2017 was developed by ISO and IEC (international electrotechnical commission) under the authority of CASCO (Committee on conformity assessment) and ISO.
This standard has high importance for IEC conformity assessment community because it states basic requirements for calibrating testing within conformity assessment schemes and programmes operating within IECEx, IEXEE, and IECRE conformity assessment systems.
References:
- URL: https://www.scc.ca Title ISO/IEC 17025 Standards Council of Canada – Conseil canadien des norms
- URL https://asq.org, Quality Management System | ASQ. ISO 9001:2015 – Quality management
- QAQC Content ISO 9001 Benefits, ISO Review, 03hadjicosts ISO 9000, 02Kaus ISO 17025, ISO standards and accreditation, Quality management, FAQ ISO 17025 and ISO 9000 by sanja lukic
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



